![Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/content_ck_images/ck_599e6db7e641a.png)
Draw the CFT diagram for [Ni(H2O)6]2+ (NO LINKS STRICTLY)!!! - Chemistry - Coordination Compounds - 11664449 | Meritnation.com
![Triplet Electronic States in d2 and d8 Complexes Probed by Absorption Spectroscopy: A CASSCF/CASPT2 Analysis of [V(H2O)6]3+ and [Ni(H2O)6]2+ | Inorganic Chemistry Triplet Electronic States in d2 and d8 Complexes Probed by Absorption Spectroscopy: A CASSCF/CASPT2 Analysis of [V(H2O)6]3+ and [Ni(H2O)6]2+ | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/ic0010860/asset/images/ic0010860.social.jpeg_v03)
Triplet Electronic States in d2 and d8 Complexes Probed by Absorption Spectroscopy: A CASSCF/CASPT2 Analysis of [V(H2O)6]3+ and [Ni(H2O)6]2+ | Inorganic Chemistry
![Assertion A solution of `[Ni(H_(2)O)_(6)]^(2+)` is green but a solution of `[Ni(CN)_(4)]^(2+)` is - YouTube Assertion A solution of `[Ni(H_(2)O)_(6)]^(2+)` is green but a solution of `[Ni(CN)_(4)]^(2+)` is - YouTube](https://i.ytimg.com/vi/nIpwGgykAQU/maxresdefault.jpg)
Assertion A solution of `[Ni(H_(2)O)_(6)]^(2+)` is green but a solution of `[Ni(CN)_(4)]^(2+)` is - YouTube

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes
✓ Solved: Describe the distribution of d electrons in [Ni(H2O)6]^2+, using crystal field theory. How...
![SOLVED: The compound [Ni(H2O)6]Cl2 is paramagnetic. Determine the oxidation number of nickel in this compound, the most likely geometry of the coordination around the nickel, and the possible configurations of the d SOLVED: The compound [Ni(H2O)6]Cl2 is paramagnetic. Determine the oxidation number of nickel in this compound, the most likely geometry of the coordination around the nickel, and the possible configurations of the d](https://cdn.numerade.com/ask_previews/543ac424-a25c-42c6-88c8-ae8f1d1a07d2_large.jpg)
SOLVED: The compound [Ni(H2O)6]Cl2 is paramagnetic. Determine the oxidation number of nickel in this compound, the most likely geometry of the coordination around the nickel, and the possible configurations of the d
![Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution. Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution.](https://d1hj4to4g9ba46.cloudfront.net/questions/2015518_249889_ans_bbd2828107e04c46b63fdcb0f0c04801.jpg)
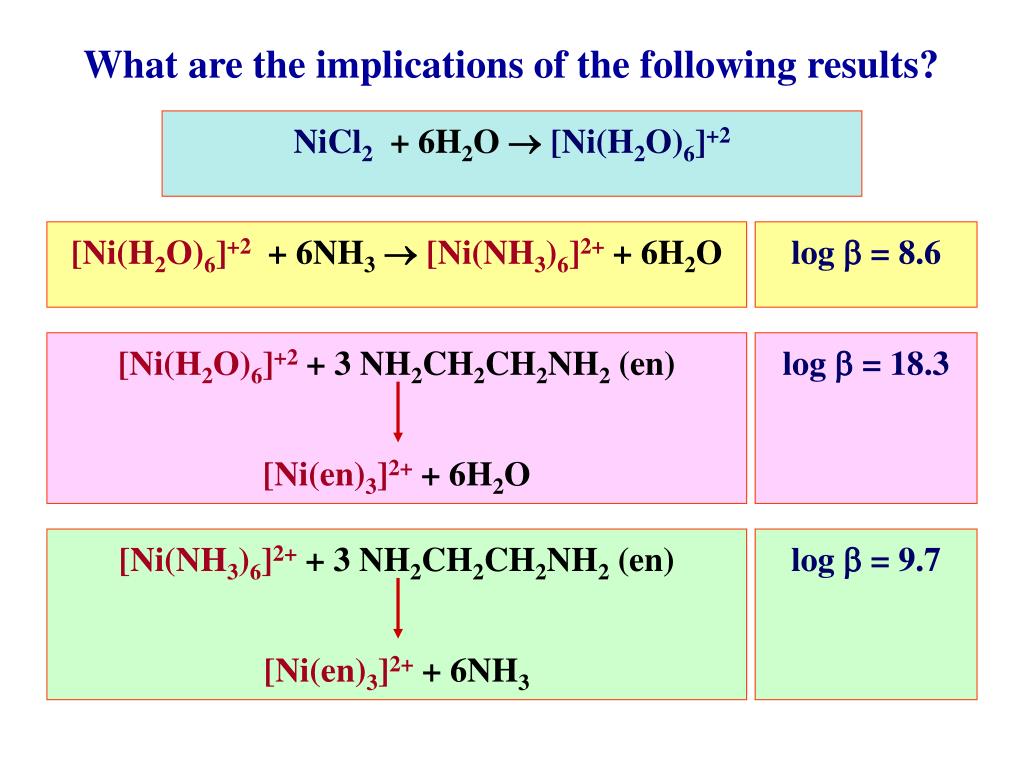
Aqueous solution of Ni^2 + contains [Ni(H2O)6]^2 + and its magnetic moment is 2.83 BM . When ammonia is added in it, comment on the magnetic moment of solution.
2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect Crystal structure and properties of the precursor [Ni(H2O)6](HTBA)2·2Н2О and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe) - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0277538713008310-fx1.jpg)
![Solved (a) The Molecular Orbital Diagram for [Ni(H2O)6]2+ is | Chegg.com Solved (a) The Molecular Orbital Diagram for [Ni(H2O)6]2+ is | Chegg.com](https://media.cheggcdn.com/study/284/28408777-e2bb-4645-8742-fab9e76a7424/image)
2 with gaseous NH3; crystal growth via in-situ solvation - ScienceDirect Reaction of [Ni(H2O)6](NO3)2 with gaseous NH3; crystal growth via in-situ solvation - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S0022024814007805-gr3.jpg)
2 and [Ni(D2O)6](ClO4)2 at Various Temperatures | Semantic Scholar FIR Spectra of [Ni(H2O)6](ClO4)2 and [Ni(D2O)6](ClO4)2 at Various Temperatures | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ecaf6b0fee69dc0a5060cdb8d7e5d0c7d395a06e/4-Table1-1.png)
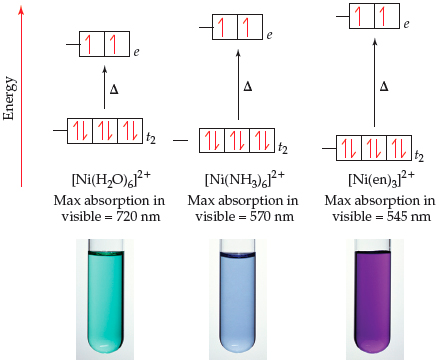
![Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram Absorption spectra of [Ni(H 2 O) 6 ] 2+ and [Ni(NH 3 ) 6 ] 2+ in... | Download Scientific Diagram](https://www.researchgate.net/publication/228364596/figure/fig3/AS:667854408003587@1536240307249/Absorption-spectra-of-NiH-2-O-6-2-and-NiNH-3-6-2-in-aqueous-solution-The.png)
![A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain. A solution of [Ni(H2O)6]^2 + is green but a solution of [Ni(CN)4]^2 - is colourless, Explain.](https://i.ytimg.com/vi/HY7e_C1lrgo/mqdefault.jpg)